Product Engine

Delivering a late-stage pipeline
Leveraging our proprietary product engine, we have demonstrated the ability to rationally select defined bacterial consortia that have gone on to show clinical activity, and generated what we believe is the most advanced portfolio of defined bacterial consortia in the industry.
Our product engine consists of our discovery platform and advanced manufacturing capabilities, supported by broad foundational intellectual property. It includes one of the largest libraries of strains of gut bacteria in the world, vast clinical datasets, proprietary capabilities in consortium design, and end-to-end CGMP manufacturing capabilities at commercial launch scale.
Together, these capabilities enable us to select defined consortia with optimal activity in a particular disease, and to generate and manufacture new product candidates repeatedly and efficiently.
Proven proprietary product engine
Interrogate human interventional FMT data to identify bacteria that correlate with clinical response
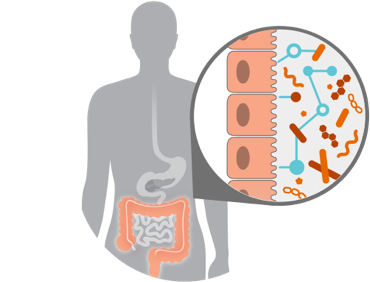
Evaluate role of microbiome in driving desired phenotype
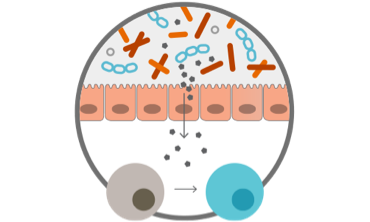
Screen one of the world’s largest microbiome strain libraries* in proprietary assays to select strains with desired pharmacology

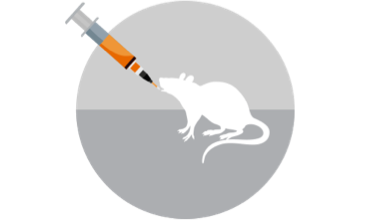
Assemble and optimize customized consortia using computational tools, co-culture systems, and animal models
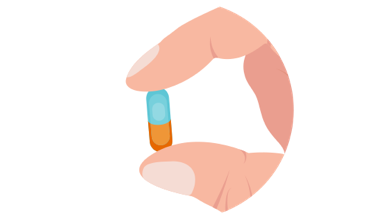
Manufacture CGMP-grade drug supplies from clonal cell banks at in-house, state-of-the-art facilities

Test defined consortia in humans to evaluate engraftment, safety, & efficacy
We believe that our product engine, including our in-house CGMP manufacturing capabilities, will allow us to successfully develop, commercialize, and maximize the impact of our novel oral therapies for patients suffering from serious GI diseases.
*Nearly 100,000 bacterial isolates obtained from hundreds of individuals across four continents

Manufacturing
Our therapeutic candidates are manufactured in our in-house CGMP manufacturing facilities.